Knee Osteoarthritis Non-Surgical Treatment Trends
A deep dive into the 2025-2026 landscape: How research volume, study quality, and payer policies are shifting for PRP, Viscosupplementation, and Corticosteroids.
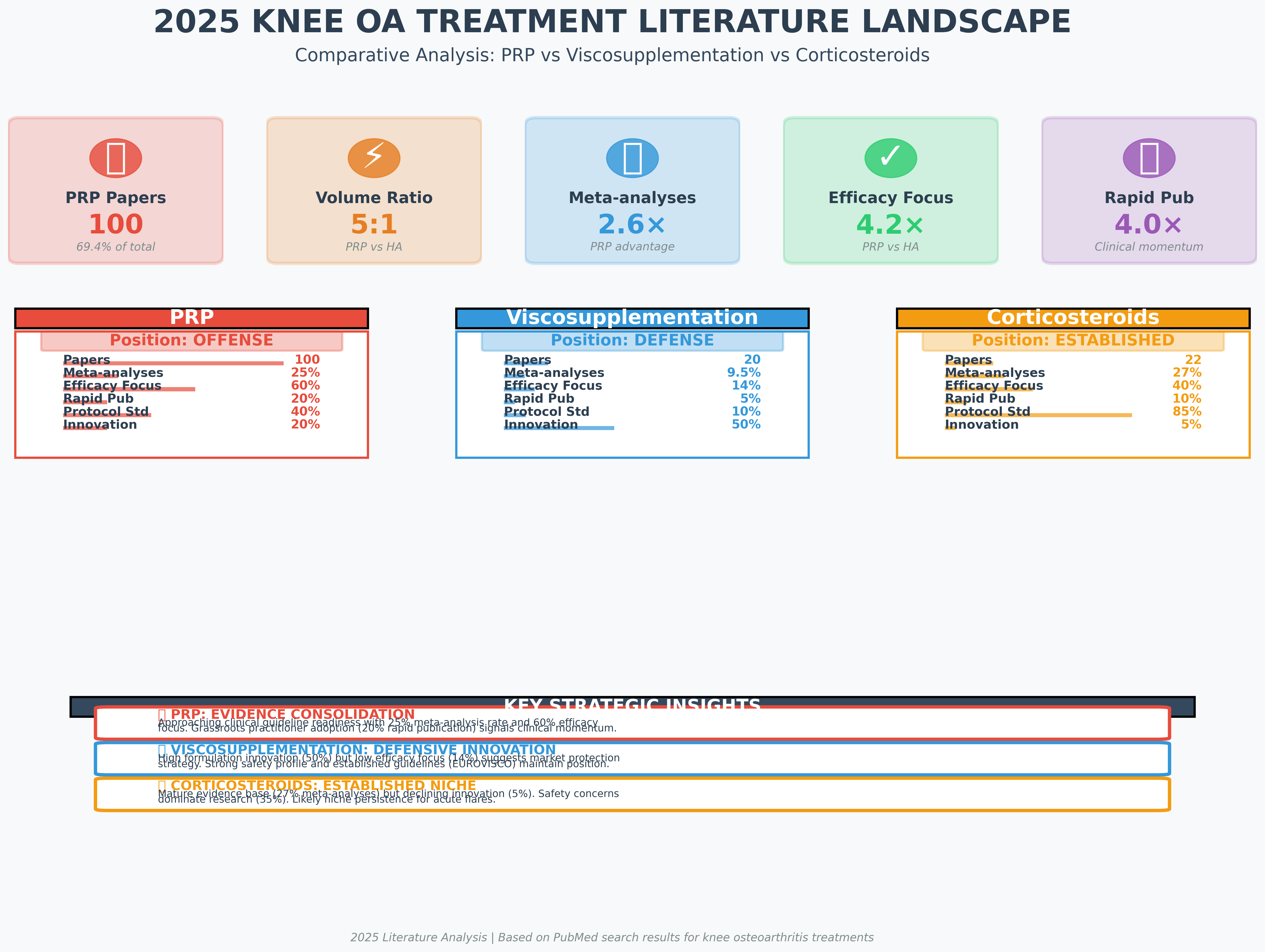
Strategic Summary: Positioning the "Big Three" non-surgical interventions based on current data trends.
The Volume Disparity
An analysis of 142 papers from 2025 PubMed searches reveals that Platelet-Rich Plasma (PRP) is currently the dominant focus of research. With 100 analyzed papers, PRP maintains a 5:1 volume ratio over Viscosupplementation (HA).
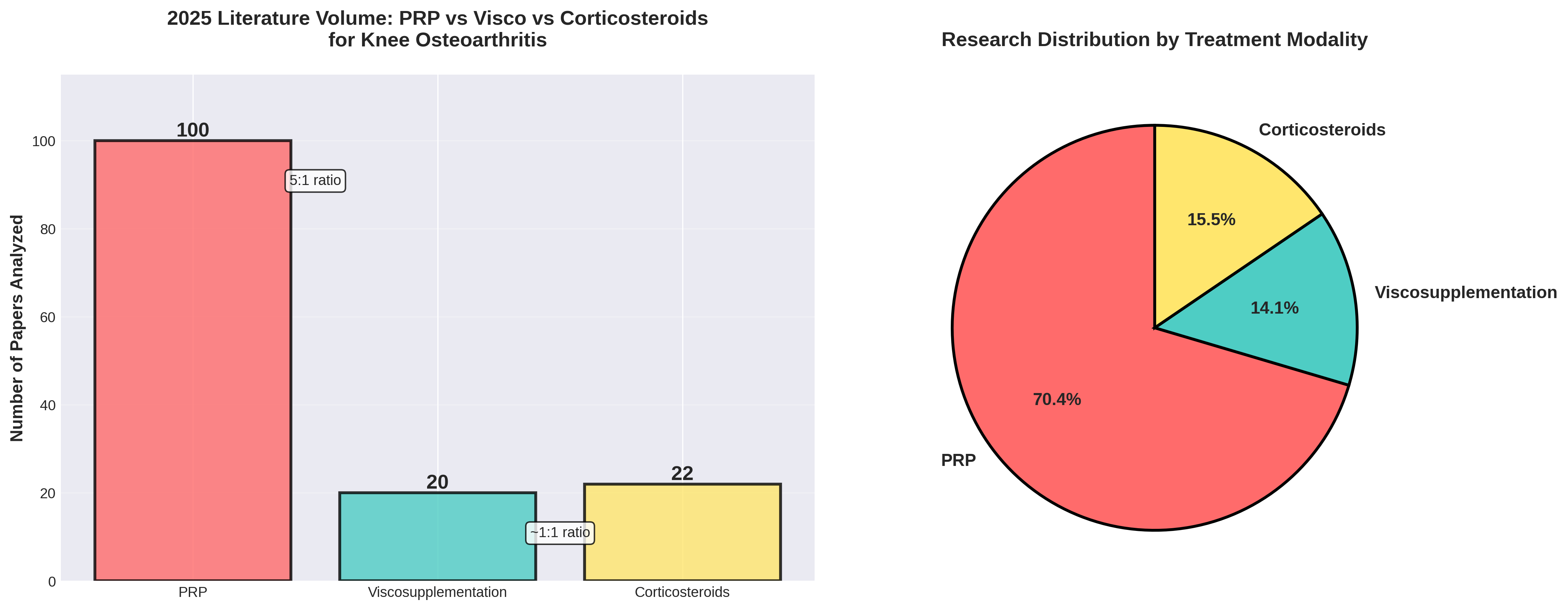
PRP accounts for over 70% of the analyzed literature, characterized by a high volume of meta-analyses (25%) indicating an evidence consolidation phase.
Research Focus: Directional Psychology
Research questions reveal the strategic mindset behind each modality. PRP is currently on the "offense," focusing heavily on efficacy (60%), while HA is in a "defensive innovation" phase, focusing on novel formulations (50%). Corticosteroids remain "established" but stagnant, with research centered primarily on safety and niche persistence.
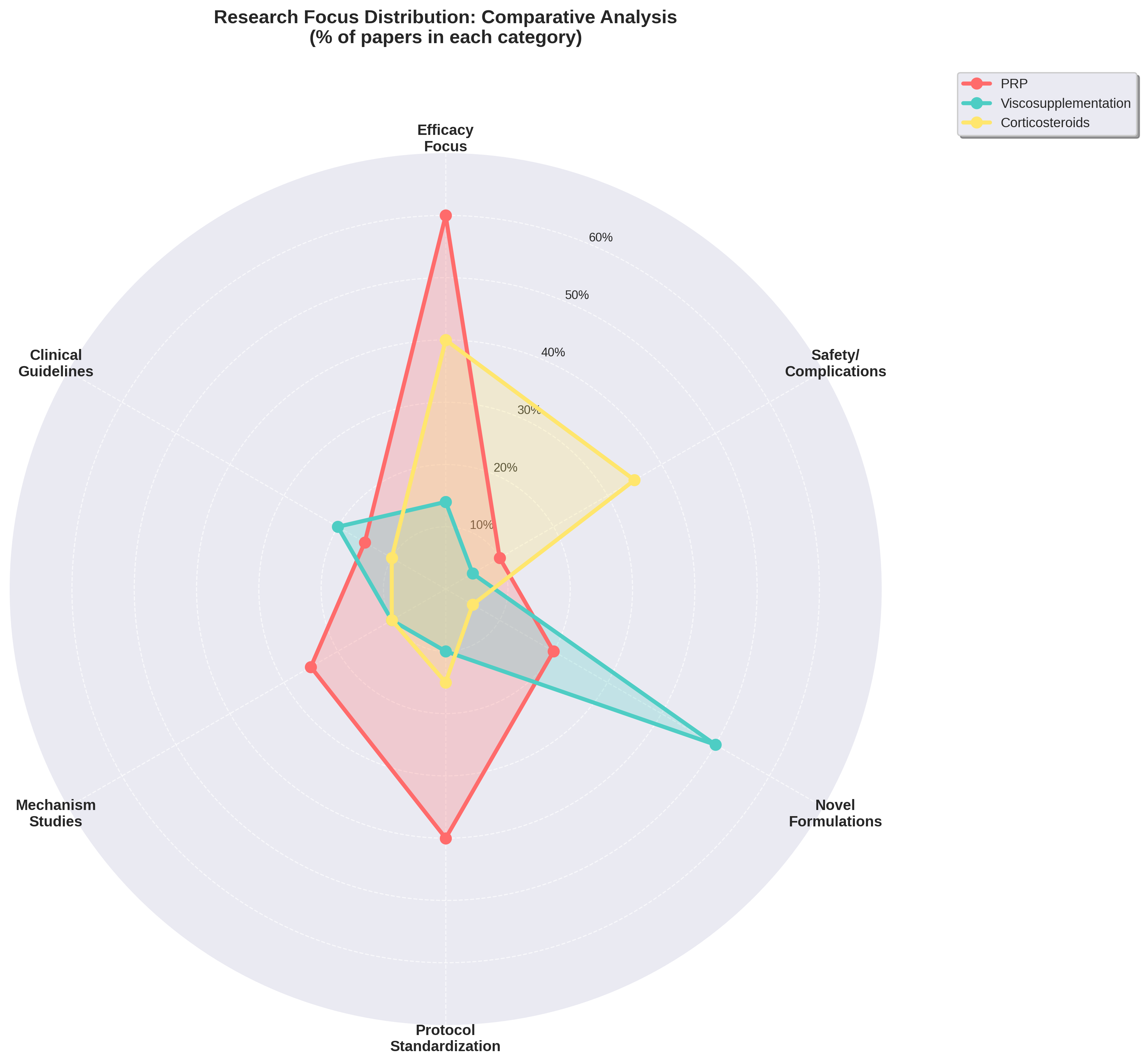
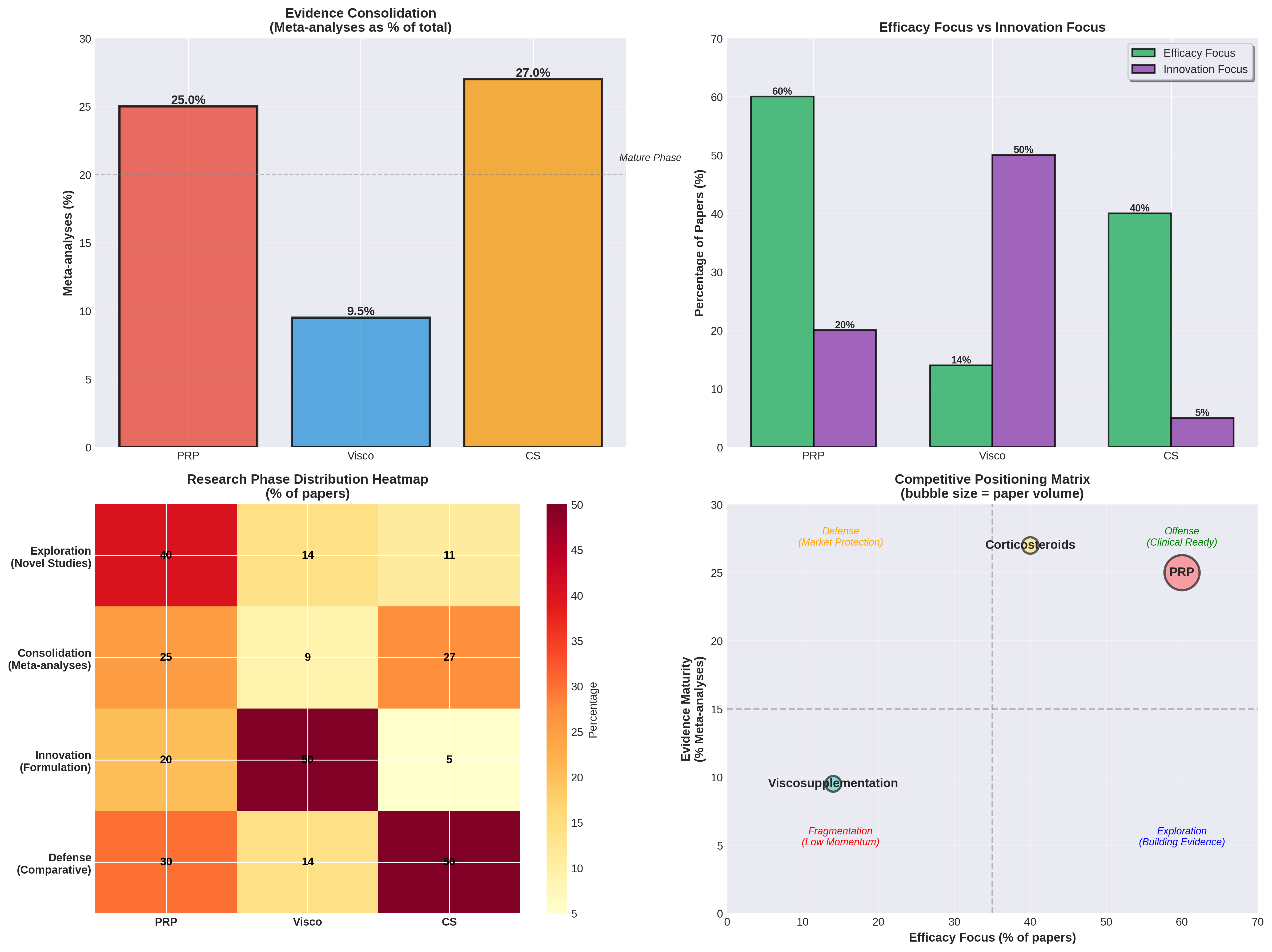
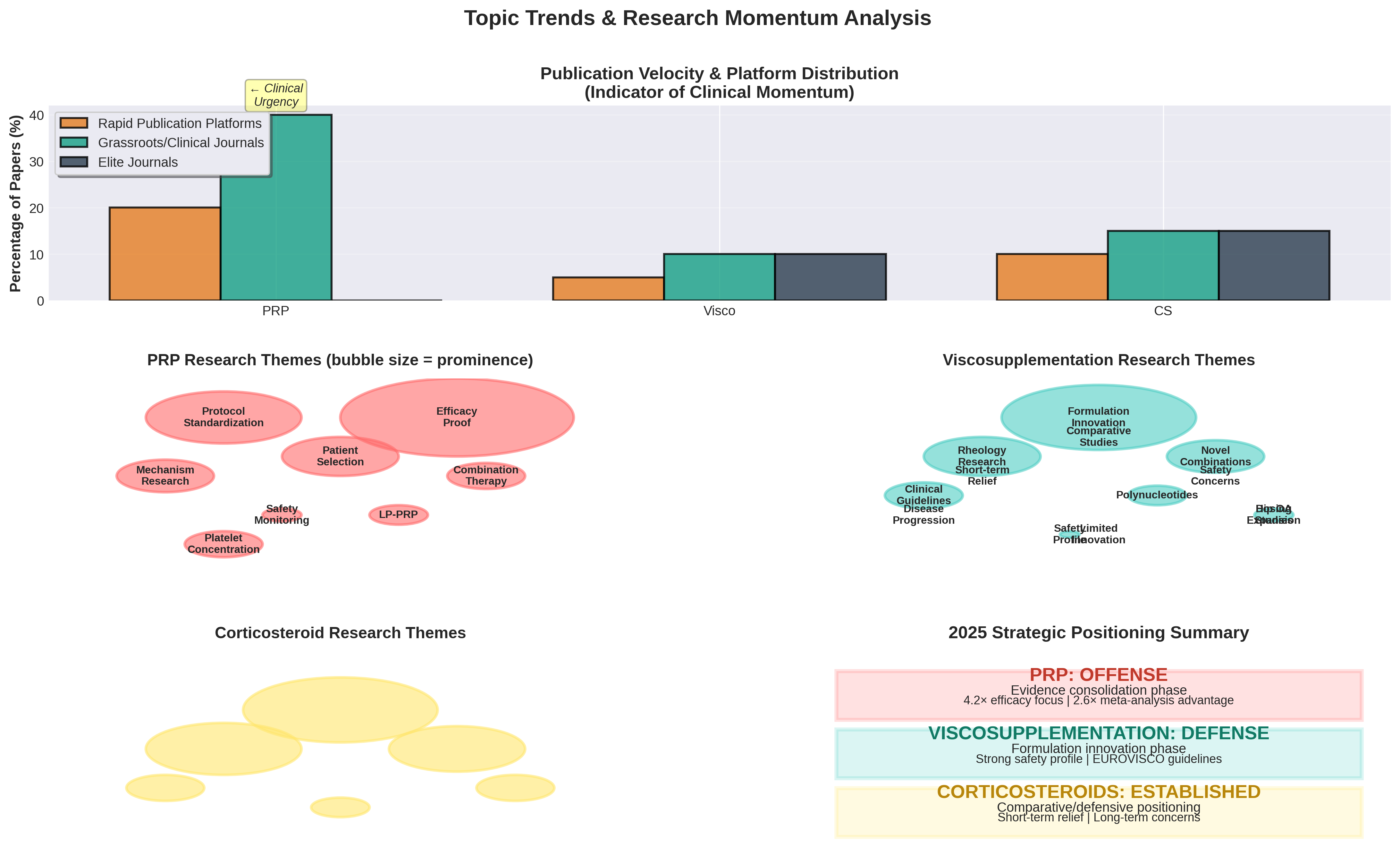
Strategic Positioning: PRP is clinical-ready and consolidating evidence; HA is focused on market protection through innovation.
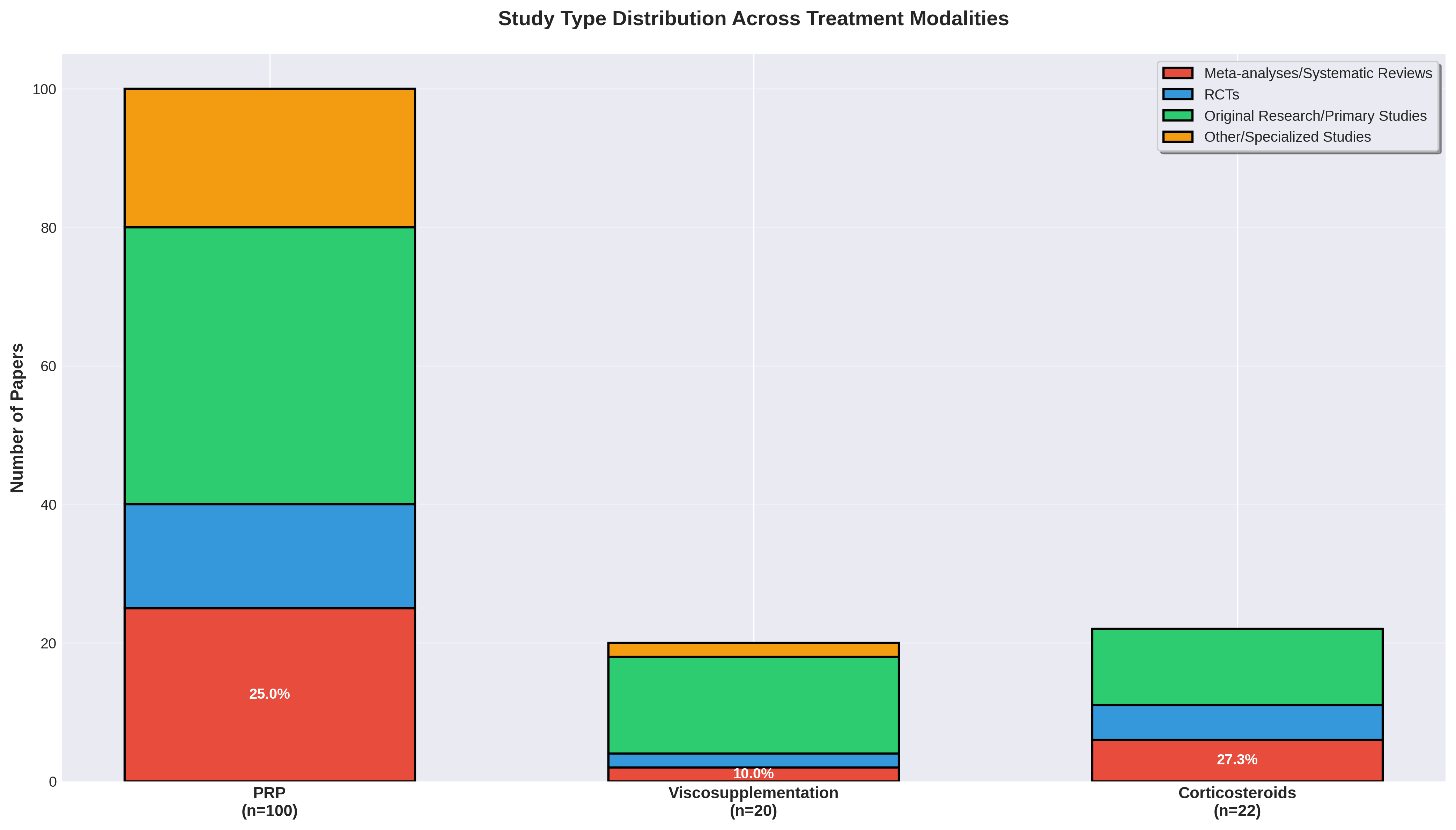
Evidence Quality & Standardization Gaps
Despite high volume, PRP continues to face challenges with protocol standardization. In contrast, while Viscosupplementation and Corticosteroids have lower research volume, they maintain more established standardization and significant presence in elite journals.
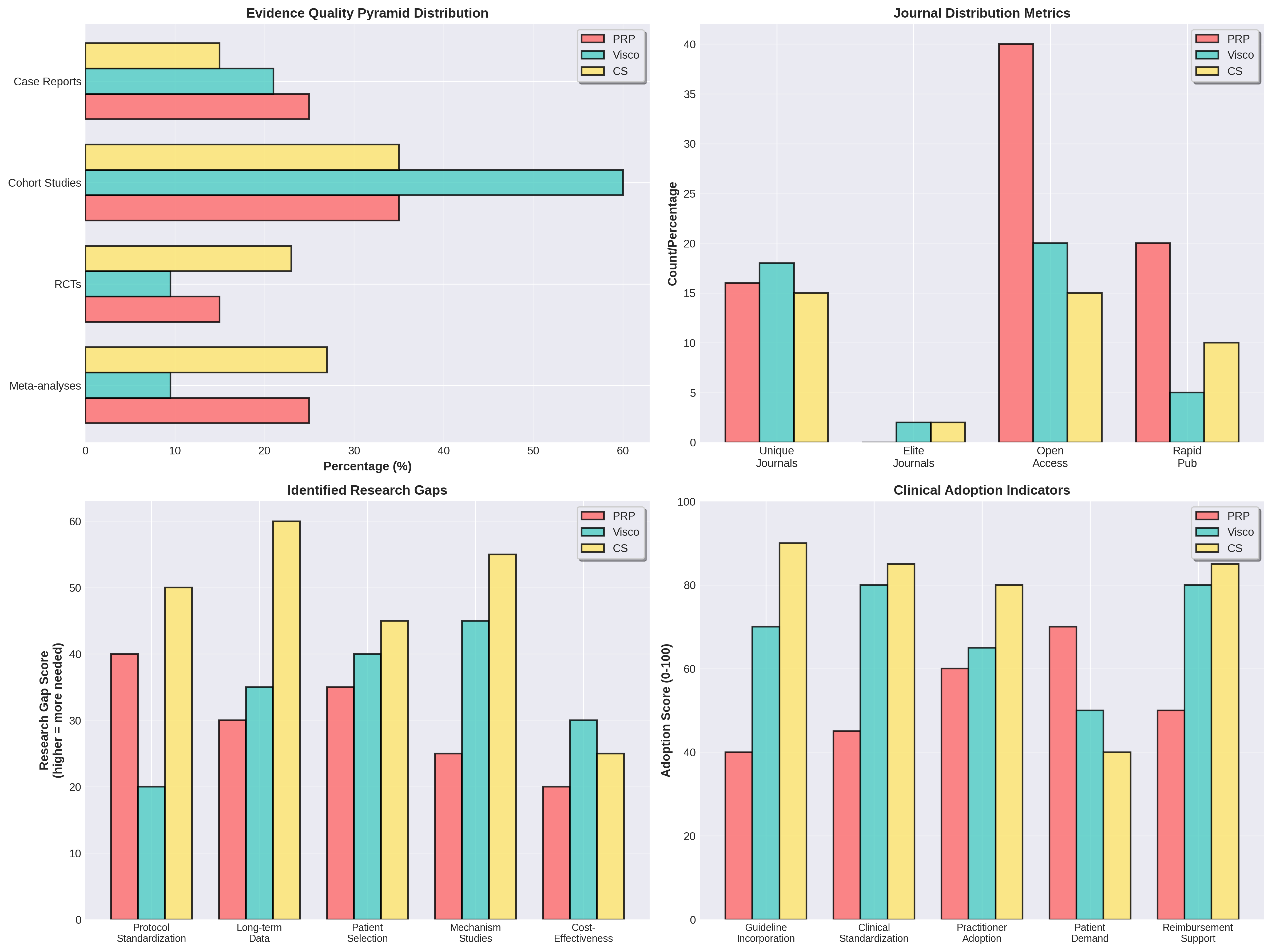
Identified Gaps: PRP research requires more protocol standardization and long-term data to match the maturity of established guidelines.
The Regulatory & Payer Disconnect
A significant gap exists between research momentum and real-world clinical business implementation. While PRP is "approaching guideline-readiness" in the literature, current U.S. payer policies for 2024-2025 largely classify it as experimental or investigational.
U.S. Payer Policy Snapshot (2024-2025)
| Payer | PRP Coverage Status | Requires CS Failure Before HA? |
|---|---|---|
| TRICARE | YES (Provisional Program) | N/A |
| Medicare | NOT Covered | Yes (Documented in LCDs) |
| Molina Healthcare | NOT Covered | Yes (At least 2 injections) |
| Blue Shield of CA | NOT Covered | Yes (Documented inadequate response) |
| Aetna / UHC | NOT Covered | No (Not mandated) |
Strategic Outlook for 2026
- PRP: Moving toward clinical-readiness. The focus on efficacy (4.2x vs HA) and evidence consolidation suggests a push for formal guideline inclusion.
- Viscosupplementation: Differentiating through rheology and novel combinations (like PN blends) to protect market position.
- Corticosteroids: Retaining a niche for acute flares, but with minimal innovation (5%) and growing safety concerns in long-term data.
The disconnect between clinical research and payer policy remains the primary challenge for practitioners designing a sustainable regenerative medicine practice.
Methodology: Comparative analysis of 142 papers from 2025 PubMed searches and U.S. payer policy documents (2023-2025).